Blatchley Lab for Engineering Dynamic Tissue Microenvironments
We seek to CHARACTERIZE tissues and organs in order to CONSTRUCT better models of human development and disease
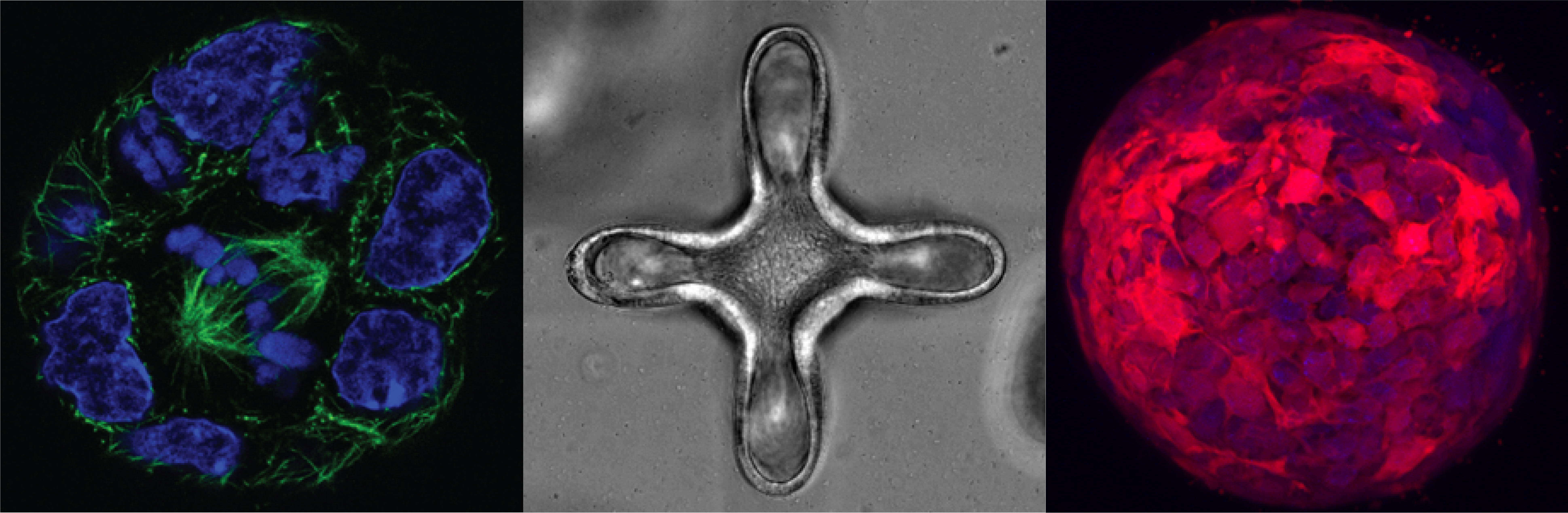
Our key tools and technologies
We use engineering tools to understand how the role of the extracellular matrix in tissue homeostasis and regeneration, as well as in development and disease.
Designer cell niches
We use existing data and new characterization methods to define the key properties of tissue-specific niches and re-build them ourselves.
Light-guided tissue engineering
We spatiotemporally tune the properties of the engineered microenvironment to deterministically guide tissue morphogenesis.
Advanced imaging techniques
We co-opt and refine analytical methods to understand cell behavior in 4D.
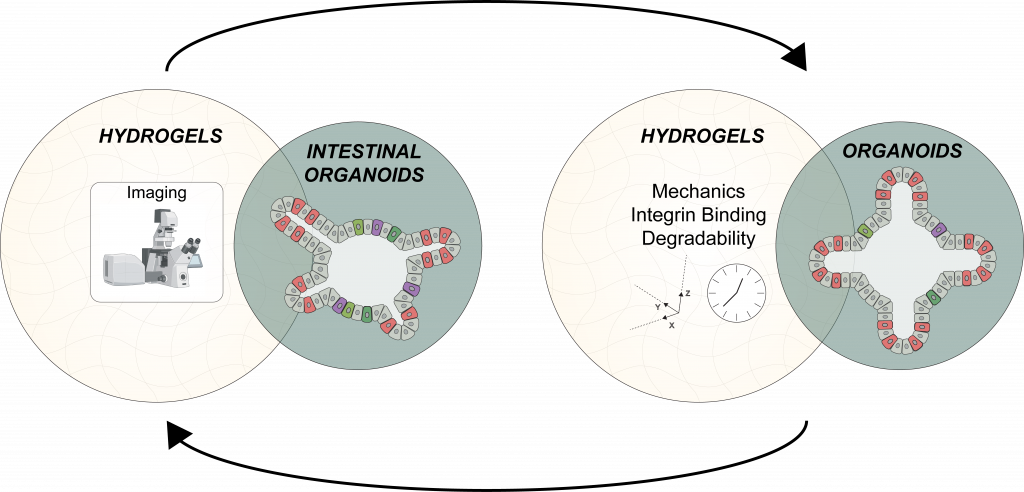


What we’ve done so far
O2-controllable materials for vasculogenesis
Stiffness controlled materials for organoids
What we’ve done so far
Photo-tunable materials to guide intestinal organoid morphometrics
What we’ve done so far
Photoexpansion microscopy (PhotoExM)
Metabolic labeling of organoid-secreted nascent proteins
What we’re doing now
Building healthy and diseased intestinal niches
Optimizing intestinal developmental niches
What we’re doing now
Studying the role of spatiotemporally variable matrix properties on intestinal development and tissue maturation
What we’re doing now
Live imaging of organoid morphogenesis
Understanding ECM dynamics
News
What’s going on with us?

Dom joins the lab as a Research Technician!
7/15/24
- Dom comes in with organoid experience, having worked with Jason Spence and Michael Dame at the University of Michigan in the Translational Tissue Modeling Laboratory
- He’ll continue work with intestinal organoids and play a major role in getting the lab up and running!
The Blatchley Lab Opens!
4/1/24
- The lab officially opened on 4/1/24 (not a joke!)
- Excitement is palpable (see photo).
- Paperwork was filled out. Emails were sent.


R00 is officially approved for funding!
3/22/24
- We’ll continue our work on iPSC-derived intestinal organoids in phototunable materials.
- Abstract here